2 feb. 2024
Enrique Gabamdé-Rodrigez, Gonzalo Soto-Heredero, et al. (BioRxiv)
Keywords
CD4+ CTL
Granulopoiesis
CCL5/CCR5 axis
Main Findings
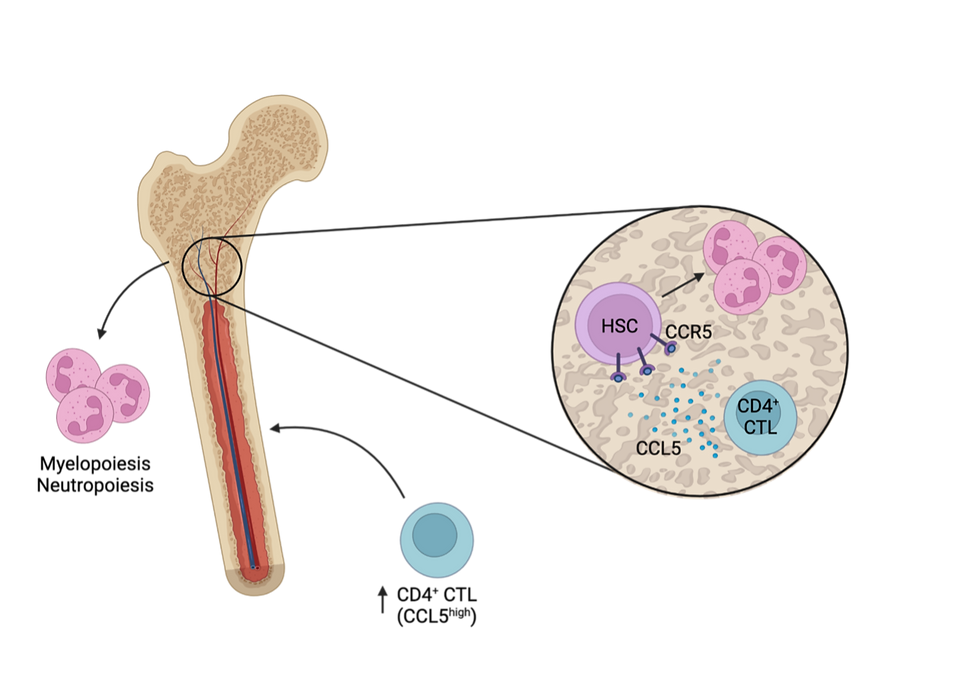
Ageing is associated with impaired immune cell function and inflammaging, a state of persistent inflammation, known to exacerbate age-associated disorders. During ageing granulopoiesis is favoured, leading to a high neutrophil to lymphocyte ratio (NLR), a predictor of mortality in age-related diseases. While healthy neutrophils are efficient in eliminating pathogens, aged neutrophils can become hyperactive and promote tissue damage. In this pre-print, Gabamdé-Rodrigez and Soto-Heredero, et al. show that CD4 T cells with a cytotoxic lymphocyte phenotype (CTLs) infiltrate the BM during ageing, and increase granulopoiesis through a targetable CCL5/CXCR5 axis.
The authors used multiparametric flow cytometry to compare different immune populations in young (3 months old) and old (24 months old) wild-type mice. They observed a higher NLR and increased pro-inflammatory circulating neutrophil population in old mice, which was confirmed to be a consequence of the aged mouse environment. Analysis of BM cells showed infiltration of a high number of CD4+ T cells in old mice. Of interest, they identified a population of cytotoxic CD4 cells exclusively in old mice which expressed CCL5, CD38 and perforin. Since the CCL5/CCR5 axis is known to be involved in myeloid skewing, the authors hypothesized that the identified CCL5+ CTL population in the old BM could be involved in the increased NLR observed in these mice. By analysing the hematopoietic compartment, they observed an upregulation of CCR5 in hematopoietic stem cells (HSCs) of old mice. The CCR5+ HSC gave rise to an increased percentage of myeloid precursors in mixed BM chimeras when compared to CCR5-/- HSCs, and overall increased the NLR in old mice. To try to revert the aged phenotype, the authors administered Maraviroc, an FDA-approved CCR5 antagonist. When compared to the vehicle administration, Maraviroc led to a decrease of the levels of myeloid progenitors and neutrophils in old mice to those observed in young mice. Moreover, the treatment resulted in an improvement of age-associated physiological parameters: reduced inflammageing-associated cytokines, increased respiratory exchange ratio and improved locomotor coordination.
In this study Gabamdé-Rodrigez and Soto-Heredero, et al. show that when exposed to an aged environment, CD4 T cells acquire a CTL phenotype expressing high levels of CCL5 and accumulate in the BM. These cells promote granulopoiesis by CCL5/CCR5 signalling with hematopoietic precursors, which leads to an increase in the number of pro-inflammatory neutrophils and an overall increased NLR ratio. By dissecting the mechanisms of how T cells disrupt the bone marrow niche during ageing, this pre-print highlights a promising therapeutic target to improve age-related disorders.
Limitations
The study is generally well designed, making good use of different genetic mouse models and adoptive transfer experiments. Additionally, the authors cleverly use spectral approaches to determine new cell populations of interest which they validate well.
While the study is a good basis, some additional points could be addressed to improve the impact and conclusiveness of the present study:
Some additional experiments to validate the role of CD4-derived CCL5 in skewing of the bone marrow would strengthen their narrative. Ideally, a CD4-T cell specific CCL5-KO would be used for such an experiment. This could for instance be done using a CD4-Cre driver or using CRISPR editing of aged CD4 T cells prior to adoptive transfer.
Additionally, it remains largely elusive which other bone marrow cells may produce CCL5. It would have been interesting to analyse the BM stromal compartment.
In our opinion, the impact of deteriorating mitochondria on the CD4+ CTL fate remains still somewhat unconnected. While chronic antigen stimulation seems to upregulate CCL5 in an in vitro system, it remains unclear whether the mitochondrial dysfunction is directly responsible for this upregulation (ie. could it be rescued using mito-protective treatments such as NAC?). The authors may consider further dissecting the implication of mitochondrial dysfunction in the development of these T cells.
Additionally, it would be interesting to study how CD4+ T cell metabolism is shifted by mitochondrial dysfunction and whether autophagy, known to decrease in many aged immune cells, may be affected
In figure 3 the authors claim that prolonged TCR engagement in an aged environment leads to the expansion of CCL5-expressing CD4+ T cells. Since the current comparison seems to not reach a significant increase in vivo, it would be ideal to increase the number of analysed mice. Moreover, assessing TCR-dependent genes (like Nur77) would aid to further confirm an increased TCR signalling.
Lastly, the study would gain more significance if the authors manage to find evidence for the existence of this pathway in humans as well.
Some small additional suggestions would be to add the statistical analysis of the figures to the corresponding figure legends, which would make it easier for reader to assess the appropriateness of the used statistical test. Moreover, the authors should consider including absolute numbers of immune cells instead of frequencies to clearly show how abundancies are shifting during aging.
Significance/Novelty
With an ever-aging population, we require more mechanistic insights to why we observe the characteristic myeloid bias in aged hematopoiesis. The authors provide an intriguing new explanation for this swaying of hematopoiesis and also provide an exciting new drug target to ameliorate this bias during aging. Targeting the CCL5-CCR5 axis using already FDA approved drugs could show an interesting new avenue to reduce some age associated comorbidities associated with inflammaging. It remains to be seen whether this axis is of equal relevance in human settings and whether it could indeed translate into a prospective anti-aging drug.
Credit
Reviewed by Felix Richter and Marta Nogueira as part of a cross-institutional journal club between the Vanderbilt University Medical Center (VUMC), the Max-Delbrück Center Berlin, the Medical University of Vienna and other life science institutes in Vienna.
The author declares no conflict of interests in relation to their involvement in the review.